12314
Views & Citations11314
Likes & Shares
Tissue damage is unavoidable during surgery and hence pain is inevitable. Successfully managing post-surgical pain and maintaining an appropriate level of analgesia is critical to a patient’s recovery. Nevertheless, post-operative acute pain management remains a challenge. In this review, we discuss several of the effective analgesia for post-operative pain management and their benefits and disadvantages.
Even though they carry multiple, significant side effects and they are associated with dependency, opioids still remain the mainstay in post-operative pain management. Besides opioids, epidural analgesia also remains a widely used analgesic treatment option. However, in the last five years, it has become more and more clear that the advantages of this technique are not as impressive as formerly believed and the disadvantages are potentially greater than estimated in the past.
Alternatively, anesthetics can be administered locally or even directly at the wound to block pain transmission from afferent nociceptive barrage. The continuous infusion of a local anesthetic, either by continuous surgical site infusion (CSSI) or continuous peripheral nerve block (CPNB), provides a constant flow of medication to the affected area, preventing gaps in treatment often seen with alternative pain management methods. Not only has the technique a proven efficacy and reduced occurrence of adverse events as seen with opioids and epidural analgesia, it may also result in an improved recovery time and earlier discharge from hospitals.
Conclusively, continuous infusion (either by CCSI or CPNB) with a local anesthetic represents a suitable and effective alternative or adjunct to existing analgesic techniques within a multimodal approach. The recent registration of a new local anesthetic solution (containing 250 ml ropivacaine 0.2%) for the treatment of acute post-operative pain can bring a ready-to-use alternative in health care.
ACUTE POST-OPERATIVE PAIN, INHERENT TO SURGICAL INTERVENTIONS
Tissue damage is unavoidable during surgery and consequently, pain inevitably occurs soon after as a result of stimulation of the nociceptors. In the 2003 survey published by Apflebaum et al. [1], we read that approximately 80% of the patients experienced acute pain after surgery of which 86% had moderate, severe or extreme pain. Moreover, experiencing postoperative pain was the most common concern (59%) of patients.
POST-OPERATIVE PAIN MAY HAVE A NEGATIVE IMPACT ON PATIENT OUTCOMES AND EXPERIENCE
The aim of post-operative pain management is to minimize patient discomfort, facilitate early mobilization and functional recovery [5]. Poor management of post-operative acute pain can contribute to medical complications which can include pneumonia, deep vein thrombosis, infection and delayed healing, as well as the development of chronic pain [6,7]. The estimated mean incidence of chronic postoperative pain is high and varies between 10 and 50% [8]. Moreover, unrelieved pain after surgery can also interfere with sleep and physical functioning and can negatively affect a patient's well-being on multiple levels [9]. Careful management of post-operative pain, on the other hand, can reduce the incidence of perioperative complications, mortality and health care costs [10]. Additionally, adequate post-operative pain management increases the overall psychological well-being of the patient [11,12].
POST-OPERATIVE PAIN MANAGEMENT: FROM SYSTEMIC TO LOCAL
For the treatment of acute post-operative pain, a range of analgesic options exist, either by systemic, regional or local analgesic techniques.
· Systemic analgesia includes NSAIDs and paracetamol (either orally or by IV) and opioids (either orally, by bolus IV or by PCA pump [1])
· Regional analgesia includes the epidural administration (EDA [2]) of local anesthetics and opioids.
· Local analgesia includes either bolus infiltration or continuous infusion of a local anesthetic.
Over time, an evolution can be noted in postoperative analgesic techniques from systemic to local applications, in view of the risk-benefit consideration [2].
MULTIMODAL ANALGESIA, A LOGIC APPROACH WITH CLINICAL BENEFITS
Pain involves multiple mechanisms that rely on different receptor systems. Based on this knowledge already in 1993, Kehlet and Dahl described the multimodal approach to achieve pain relief in the postoperative setting [13].
Multimodal pain management uses two or more pain treatments that act by different analgesic mechanisms, at different sites in the nervous system and at lower doses to achieve additive or synergistic effects [14]. By combining the various analgesic techniques, multimodal therapy aims to provide effective postoperative pain relief, reduce opioid-related adverse effects, reduce surgical stress response and improve clinical outcomes, without increasing adverse events compared with increased doses of single agents [15].
OPIOIDS AS AN ANALGESIC TREATMENT OPTION
Opioid analgesics are still the mainstay in post-operative pain management [3]. However, whether delivered orally, via PCA or in an injectable format, opioids carry multiple, significant side effects, including nausea, vomiting, constipation, over sedation, somnolence and respiratory depression [2]. Additionally, there is the potential threat of dependency (in up to 15% of the patients) which could already be induced as of 10 days after the initial treatment [16]. The excessive use of opioids has resulted into an opioid epidemic in the USA [17,18].
EPIDURAL ANALGESIA AS ANANALGESIC TREATMENT OPTION
Epidural analgesia is a widely used technique to treat postoperative pain after major surgery and has been considered the ‘gold standard’ for decades. This is not surprising as the technique offers effective pain relief and significantly reduces morbidity and mortality [2]. However, based on current evidence, the advantages of epidural analgesia seem to be less impressive as formerly believed. Indeed, not only does epidural anesthesia seem to be associated with several (underestimated) adverse events such as nausea, hypotension, neuraxial hematoma, cardiac arrest and epidural abscess [19-21], the failure rates are also potentially higher than estimated in the past [2].
LOCAL INFILTRATION AND PERINEURAL ANALGESIA: USEFUL ADJUNCTS IN THE MULTIMODAL APPROACH TO POST-OPERATIVE PAIN MANAGEMENT
From a theoretical point of view, the administration of an anesthetic locally or even directly at the wound site is the most rational approach to reduce the barrage of signals from the nociceptors and thereby decrease the pain response [23]. The routine use of peripheral nerve blocks and wound infiltration with long-acting local anesthetics as an adjuvant to local, regional and general anesthetic techniques can improve post-operative pain management after a wide variety of surgical procedures [2,24]. Additionally, local analgesia has been associated with a reduced risk of developing chronic post-operative pain [7].
Continuous surgical site infusion (CSSI)
CSSI, also known as continuous wound infusion or CWI, is defined as a continuous infusion into the surgical incision site. A fenestrated (multi-holed) catheter may be inserted into the surgical site to distribute the local anesthetic inside the wound and provide post-operative pain control for 24-72 h.
Moreover, local anesthetics inhibit the inflammatory injury and may, therefore, reduce the risk of hyperalgesia [2].
As Raines et al. [26] explained, CSSI provides several important benefits over other currently practiced post-operative pain control techniques. Advantages of the wound infusion technique are that the infusion affects only the surgical area and eliminates the risk of vascular, pleural, or neural placement of needles, thereby allowing its use in patients in whom an epidural technique cannot safely be used (e.g. due to coagulopathy, use of thrombolytic or anatomic anomalies).
Continuous peripheral nerve block (CPNB)
CPNB is defined as a continuous infusion around a group of nerves. A catheter is inserted percutaneously adjacent to a target nerve/plexus that is most relevant to the surgical site followed by the infusion of local anesthetic through the catheter, thereby providing sensory and motor analgesia [31].
Peripheral nerve catheter analgesia has been shown to provide a significant improvement in postoperative pain control compared with opioids and a decrease in opioid-related side effects after different types of surgical procedures [32,33]. Compared to systemic patient-controlled analgesia, a 40-70% decrease of opioid use could be observed on top of a better pain control after surgery [33]. The publication by Afsari equally confirms that continuous perineural infusions provide high-quality local analgesia superior to parenteral opioid analgesia and offer equivalent analgesia but less adverse effects than epidural analgesia [34]. Therefore, it has been recommended as the preferred choice for major orthopedic surgical procedures [35,36].
In their 2015 review, Machi and Ilfeld [37] concluded that CPNB is a well-accepted and research-supported technique for the well tolerated and efficacious treatment of moderate-to-severe pain related to surgery in the ambulatory setting. As can be concluded for CSSI, also CPNB seems to allow for earlier mobilization and hence early discharge [33,38].
LOCAL INFILTRATION AND PERINEURAL ANALGESIA: OPIOID SPARING AND COST-EFFECTIVE?
As already discussed, the application of local anesthetics can reduce the need for rescue medication (systemic opioids) after surgery when administered via CSSI or CPNB.
Minimizing narcotic consumption can decrease post-operative nausea and vomiting, expedite rehabilitation, improve recovery time, and can lead to earlier discharge from hospitals [39]. Moreover, CCSI may reduce total cost of care [39-41].
[1] PCA: Patient ControlledAnalgesia
[2] EDA: Epidural Analgesia
WHAT’S NEW?
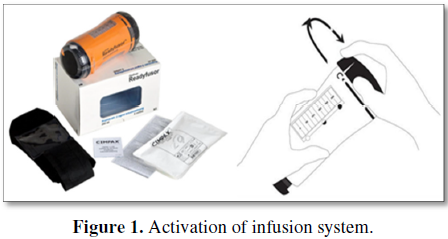
Recently AIFA approved the registration of a new medicinal product for the treatment of acute post-operative pain. Ropivacaina BioQ Readyfusor is a ready-to-use, factory-filled infusion system containing 250 ml ropivacaine 0.2%. It is indicated for the treatment of postoperative pain in adults (>18 years) and can be used for continuous surgical site infusion (CSSI) and continuous peripheral nerve blockade (CPNB). The ready-to-use administration system delivers the ropivacaine solution at 5 ml/h for 48 h. The infusion system can be activated by simply rotating the cap of the system to the “on” position (Figure 1). Within the multimodal approach, Ropivacaina BioQ Readyfusor can have its place in the post-operative pain management landscape, alongside or replacing existing treatment options.
1. Apfelbaum JL, Chen C, Mehta SS, Gan TJ (2003) Post-operative pain experience: Results from a National Survey suggest postoperative pain continues to be undermanaged. Anesth Analg 97: 534-540.
2. Rawal N (2016) Current issues in postoperative pain management. Eur J Anesthesiol 33: 160-171.
3. Gan TJ (2017) Poorly controlled postoperative pain: Prevalence, consequences and prevention. J Pain Res 10: 2287-2298.
4. Sommer M, de Rijke JM, van Kleef M, Kessels AG, Peters ML, et al. (2008) The prevalence of post-operative pain in a sample of 1490 surgical inpatients. Eur J Anesthesiol 25: 267-274.
5. Corke P (2013) Postoperative pain management. Aust Prescr 36: 202-205.
6. Meisner W, Coluzzi F, Fletcher D, Huygen F, Morlion B, et al. (2015) Improving the management of post-operative acute pain: Priorities for change. Curr Med Res Opin 31: 2131-2143.
7. Weinstein EJ, Levene JL, Cohen MS, Andreae DA, Chao JY, et al. (2018) Local anesthetics and regional anesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev 6: CD007105.
8. Schnabel A, Pogatzki-Zahn E (2010) Predictors of chronic pain following surgery. What do we know? Schmerz 24: 517-531.
9. Sinatra R (2010) Causes and consequences of inadequate management of acute pain. Pain Med 11: 1859-1871.
10. Rawal N (2001) Treating postoperative pain improves outcome. Minerva Anestesiol 67: 200-205.
11. Claroni C, Marcelli ME, Sofra MC, Covotta M, Torregiani G, et al. (2016). Preperitoneal continuous infusion of local anesthetics: What is the impact on surgical wound infections in humans? Pain Med 17: 582-589.
12. Sinatra RS, Torres J, Bustos AM (2002) Pain management after major orthopedic surgery: Current strategies and new concepts. J Am Acad Orthop Surg 10: 117-129.
13. Kehlet H, Dahl JB (1993). The value of “multi-modal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg 77: 1048-1056.
14. Wu CL, Raja SN (2011) Treatment of acute post-operative pain. Lancet 377: 2215-2225.
15. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, et al. (2016) Management of postoperative pain: A clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee and Administrative Council. J Pain 17: 131-157.
16. Wardhan R, Chelly J (2017) Recent advances in acute pain management: Understanding the mechanisms of acute pain, the prescription of opioids and the role of multimodal pain therapy. F1000Res 6: 2065.
17. Koepke EJ, Manning EL, Miller TE, Ganesh A, Williams DGA, et al. (2018) The rising tide of opioid use and abuse: The role of the anesthesiologist. Perioper Med (Lond) 7: 16.
18. Shipton EA, Shipton EE, Shipton AJ (2018) A review of the opioid epidemic: What do we do about it? Pain Ther 7: 23-36.
19. Rawal N (2012) Epidural technique for postoperative pain: Gold standard no more? Reg Anesth Pain Med 37: 310-317.
20. Horlocker T, Kopp S (2013) Epidural hematoma after epidural blockade in the United States: It's not just low molecular heparin following orthopedic surgery anymore. Anesth Analg 116: 1195-1197.
21. Pitkänen MT, Aromaa U, Cozanitis DA, Förster JG (2013). Serious complications associated with spinal and epidural anesthesia in Finland from 2000 to 2009. Acta Anesthesiol Scand 57: 553-564.
22. Paul JE, Buckley N, McLean RF, Antoni K, Musson D, et al. (2014) Hamilton acute pain service safety study: Using root cause analysis to reduce the incidence of adverse events. Anesthesiology 120: 97-109.
23. Kehlet H, Liu SS (2007) Continuous local anesthetic wound infusion to improve postoperative outcome. Back to the periphery? Anesthesiology 107: 369-371.
24. White PF (2009) The role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg 10: S5-22.
25. Liu SS, Richman JM, Thirlby RC, Wu CL (2006) Efficacy of continuous wound catheters delivering local anesthetic for post-operative analgesia: A quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 203: 914-932.
26. Raines S, Hedlund C, Franzon M, Lillieborg S, Kelleher G, et al. (2014) Ropivacaine for continuous wound infusion for post-operative pain management: A systematic review and meta-analysis of randomized controlled trials. Eur Surg Res 53: 43-60.
27. Ventham NT, O'Neill S, Johns N, Brady RR, Fearon KC (2014) Evaluation of novel local anesthetic wound infiltration techniques for postoperative pain following colorectal resection surgery: A meta-analysis. Dis Colon Rectum 57: 237-250.
28. Mungroop TH, Bond MJ, Lirk P, Busch OR, Hollmann MW, et al. (2018) Preperitoneal or subcutaneous wound catheters as alternative for epidural analgesia in abdominal surgery: A systematic review and meta-analysis. Ann Surg.
29. Rackelboom T, Le Strat S, Silvera S, Schmitz T, Bassot A, et al. (2010) Improving continuous wound infusion effectiveness for postoperative analgesia after cesarean delivery: A randomized controlled trial. Obstet Gynecol 116: 893-900.
30. Adesope O, Ituk U, Habib AS (2016) Local anaesthetic wound infiltration for post-caesarean section analgesia: A systematic review and meta-analysis. Eur J Anaesthesiol 33: 731-742.
31. Ilfeld BM (2017) Continuous peripheral nerve blocks: An update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg 124: 308-335.
32. Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, et al. (2006) Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 102: 248-257.
33. Chelly JE, Ghisi D, Fanelli A (2010) Continuous peripheral nerve blocks in acute pain management. Br J Anaesth 105: i86-96.
34. Afsari M, McCartney CJL (2010) Perineural catheter techniques. Int Anesthesiol Clin 48: 71-84.
35. Fowler SJ, Symons J, Sabato S, Myles PS (2008) Epidural analgesia compared with peripheral nerve blockade after major knee surgery: A systematic review and meta-analysis of randomized trials. Br J Anaesth 100: 154-164.
36. Luo J, Min S (2017) Post-operative pain management in the post-anesthesia care unit: An update. J Pain Res 10: 2687-2698.
37. Machi AT, Ilfeld BM (2015) Continuous peripheral nerve blocks in the ambulatory setting: An update of the published evidence. Curr Opin Anesthesiol 28: 648-655.
38. Joshi G, Gandhi K, Shah N, Gadsden J, Corman SL (2016) Peripheral nerve blocks in the management of postoperative pain: Challenges and opportunities. J Clin Anesth 35: 524-529.
39. Beaussier M, El’Ayoubi H, Schiffer E, Rollin M, Parc Y, et al. (2007) Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery. Anesthesiology 107: 461-468.
40. Forastiere E, Sofra M, Giannarelli D, Fabrizi L, Simone G (2008) Effectiveness of continuous wound infusion of 0.5% ropivacaine by On-Q pain relief system for postoperative pain management after open nephrectomy. Br J Anesth 101: 841-847.
41. Tilleul P, Aissou M, Bocquet F, Thiriat N, le Grelle O, et al. (2012) Cost-effectiveness analysis comparing epidural, patient-controlled intravenous morphine and continuous wound infiltration for post-operative pain management after open abdominal surgery. BJA 108: 998-1005.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)